Introduction
Water is the foundation of media used for mammalian cell culture and assisted reproductive technologies – its purity is central to providing optimal conditions for embryo development. While water is ubiquitous, covering over 70% of the Earth’s surface, and is vital to all forms of life, impurities in water can have deleterious effects on embryo development. When consumed, our bodies make use of valuable components water delivers and remove those that are unnecessary or harmful. Preimplantation embryos lack the complex systems required to filter impurities, making water purity a critical component of the culture media formula.
In addition to the obvious importance of water as the basis of primordial soup, providing most of the volume of the cell, water plays a key role in nearly all cellular processes. For example, water is an excellent universal polar solvent for numerous substances including inorganic salts, organic sugars, and gases such as oxygen and carbon dioxide. With a pH of 7.0, water is also central to acid-base neutrality (viz: white paper on pH) and enzyme function.
As such, water is essential to metabolism – being removed during anabolism to synthesize large molecules such as starch, triglycerides and proteins and, conversely, contributing molecules to catabolism to yield smaller molecules such as glucose, fatty acids and amino acids. Not only is water fundamental to photosynthesis and respiration, but it also enables organic compounds such as proteins, DNA and polysaccharides to influence protein folding, DNA base pairing and other phenomena crucial to the existence and replication of life.
Types of Pure Water
Fresh water is a natural product obtained from both surface water, such as rainwater, lakes and rivers, and ground water, such as aquifers, wells and springs.
Generally, sources of water at higher altitudes tend to be less polluted than those at low altitudes, with higher levels of dissolved oxygen.
Nevertheless, the purity of water from different sources varies and, in most cases, requires purification to remove common impurities such as metal salts and oxides including copper, iron, calcium, magnesium and lead, and potentially harmful microbes such as viruses, protozoa and bacteria (Table 1).
In general, there are several types of pure water used in everyday activities and in industry, medicine and science:
Deionized water: Also referred to as demineralization, fresh water is passed through cation and anion resin beds to remove almost all mineral ions, including cations such as sodium, calcium, magnesium, iron and copper, and anions such as chloride and sulfate. Since most non-particulate water impurities are dissolved salts, and because the structure of the resin beds removes most particulates, deionization yields highly pure water. However, deionization is unable to remove some contaminants such as uncharged organic molecules.
Distilled water: One of the oldest and most effective water purification methods is the simple still. Fresh water is boiled in one container and the vapor phase condenses in another container, leaving behind microorganisms and non-volatile or mineral impurities that fail to boil at or near the boiling point of water. Repeating the distillation process yields water of even greater purity, termed double-distilled water.
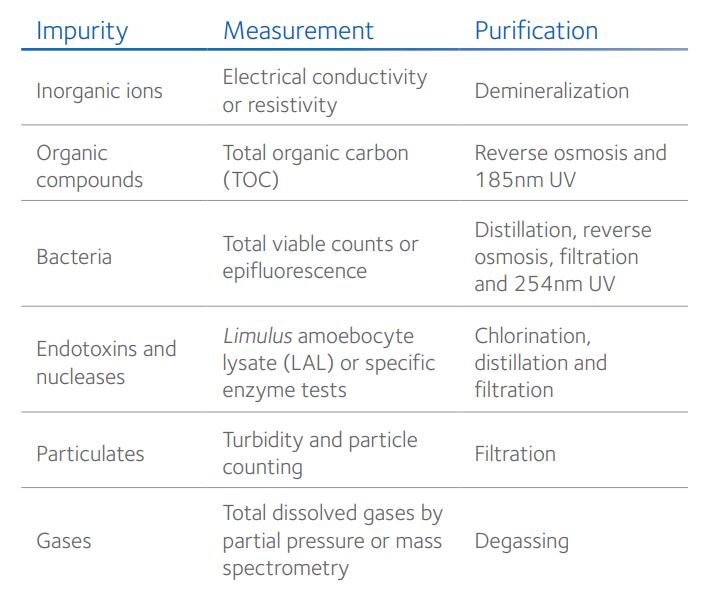
Figure 1: The CO2/ bicarbonate buffering system that is the
predominant method for establishing pHe of culture media.
Ultrapure water (UPW): Unlike deionization or distillation, ultrapurification requires several purification steps and stringent analytical measures of purity. In order to become ultrapure, fresh water undergoes several purification steps, including reverse osmosis, deionization, filtering and UV light treatment to yield high-quality water without significant contamination (i.e. <1mg/100ml elements other than water). This water is used in the semi-conductor industry and medicinally as water for irrigation and water for injection. Water for irrigation is used for applications without particulate matter specifications whereas water for injection is solute-free and used for applications that require bacterial endotoxin specifications and, therefore, may also contain bacteriostatic or antimicrobial agents. A typical system for producing UPW is comprised of three stages: a pretreatment stage to produce purified water; a primary stage to further purify the water; and lastly a polishing stage that yields UPW.
Water Purity Specifications
There are many grades of water with various standards, depending on the regulatory body. For example, the American Society for Testing and Materials (ASTM) defines water purity using a four type scale plus three sub-standards (Table 2) whereas the International Organization for Standardization (ISO) uses a three grade scale (Table 3), Type 1 and Grade 1 being the most pure water, respectively. In general, deionized or distilled water is classified as Type 2 or Type 3, whereas UPW is Type 1 water.
The defining characteristic of UPW is its resistivity, where UPW is often referred to as 18 megaohm (MΩ) water. An ohm is the amount of electricity (voltage) required to deliver 1 ampere of current. Materials used for transmitting electricity, such as copper, have ohm values in the µΩ range, and most tap water, which contains a plethora of charged ions, has a resistivity of 100-5000 Ω (0.001-0.05 MΩ).
In contrast, the resistivity of absolute pure water is 18.2 MΩ at 25°C. Water of this quality must be measured inline (closed system) in order to prevent atmospheric interference of the reading. As water is drawn from a water purification system that is showing 18.2 megaohms per centimetre (MΩ-cm) purity, carbon dioxide from the atmosphere is immediately absorbed into the solution. The carbon dioxide reacts with water forming carbonic acid in solution, resulting in an immediate decrease in the resistance.
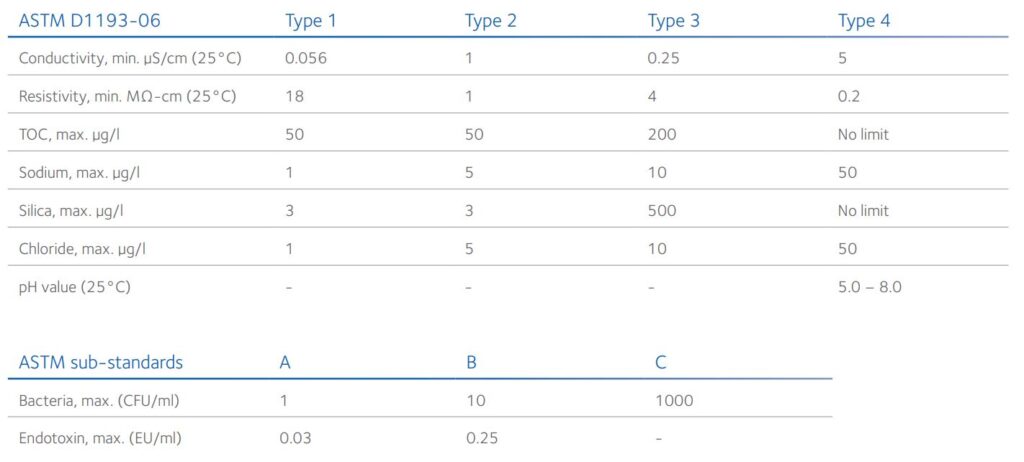
Table 2: ASTM International standard specification for reagent grade water¹
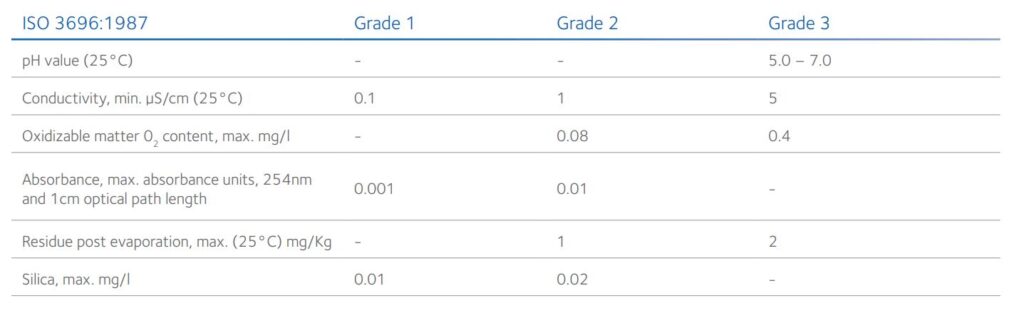
Table 3: ISO specification for analytical lab grade water²
The measurement of various parameters of water purity may be interpreted as follows:
Conductivity: The most common measure of water purity is the amount of electrical current that passes through the water. Conductivity is the inverse of resistivity. Conductivity is measured in microSiemens per centimeter (µS/cm) at a temperature of 25°C. Conductivity meters are readily available and used for testing all types of water.
Resistivity: As detailed earlier, resistivity measurements are required for ultrapure water, are typically built into the line of water purification, and yields a value that is the inverse of conductivity. Ultrapure water completely devoid of dissolved ions is essentially non-conductive and, therefore, has high resistivity.
pH: The relative acidity or alkalinity of the water depends on the concentration of hydrogen ions present (viz: white paper on pH). High purity water has no impact on pH because it is devoid of dissolved ions.
Total organic carbon (TOC): The amount of organic content present within the water is measured in parts per billion (ppb) or micrograms per liter (µg/l) and must be less than 50 ug/L. Organic compounds are naturally present within water.
Endotoxins: The amount of endotoxin within water is measured in endotoxin units per milliliter (EU/ml). Endotoxins are byproducts of the bacterial lifecycle and are also generated when using purification technologies to destroy bacteria.
Water purification for culture media

The highest specification ultrapure water is required as the basis for manufacture of modern, high quality mammalian cell culture media, in line with the European Medicines Agency and US Pharmacopeia guidelines on the quality of water for pharmaceutical use (3,4).
An example of the stringent methods required to produce ultrapure water is the purification process adopted by the CooperSurgical’s media production plant.
Located in mountainous Costa Rica, one of the most environmentally sustainable, biodiverse, rain-forested countries in the world, CooperSurgical’s water treatment and distribution system is comprised of four separate stages: pre-treatment, polishing, storage and distribution.
Pre-treatment: Municipal water from nearby water wells enters the system via a static mixer, where it is combined with sodium metabisulfite to remove chlorine. The dechlorinated water is then passed through a 5µm filter to remove large particles and a 254nm UV lamp to remove microbes. The filtered water then passes through a set of parallel ‘softeners’ to remove divalent cations such as Mg2+ and Ca2+ . The ‘softened’ water is then passed through a reverse osmosis unit to remove ions (~96% of salts in solution), as well as remaining microorganisms and smaller particulates. Polishing: Permeate from the reverse-osmosis system is passed into the ‘polishing’ unit which consists of three deionization tanks in series. Water flows over a mixed bed of cationic and anionic resins within the deionizers, exchanging charged species for H+ and OH- ions. Purified water from these tanks passes through a second 254nm UV lamp in order to eliminate any microbes that may have accumulated within the deionization tanks. The water then passes through a 1µm filter followed by a parallel set of ultrafilters with a nominal pore size of 0.03µm, designed to remove dead bacteria and viruses.
Polishing: Permeate from the reverse-osmosis system is passed into the ‘polishing’ unit which consists of three deionization tanks in series. Water flows over a mixed bed of cationic and anionic resins within the deionizers, exchanging charged species for H+ and OH– ions. Purified water from these tanks passes through a second 254nm UV lamp in order to eliminate any microbes that may have accumulated within the deionization tanks. The water then passes through a 1µm filter followed by a parallel set of ultrafilters with a nominal pore size of 0.03µm, designed to remove dead bacteria and viruses.
Storage and distribution: Following ultrafiltration, purified water flows into a large, stainless steel tank for storage and distribution. A pump pulls water from the storage tank and continuously circulates it in the distribution loop to prevent biofilm growth. Before entering the culture media production area, the circulating water passes through two parallel deionizer tanks, a set of 0.22µm filters and a set of ultrafilters with a molecular weight cut-off of 6000 Da, designed to remove bacteria and endotoxins. This second polishing step increases the resistivity of the water to meet ultrapure and water for injection specifications and ensures that any changes within the storage tank do not affect the water sent to the production area. The filtered pure water flows through a pair of 185nm UV TOC lamps on the return side of the distribution loop, eliminating any organic carbon that may have developed during distribution.
References
- ASTM International, 2018. D5127-13 Standard Guide for Ultra-Pure Water Used in the Electronics and Semiconductor Industries. [Online] Available at: https://www.astm.org/Standards/D5127.htm
- ISO, 2018. ISO 3696:1987 Water for analytical laboratory use — Specification and test methods. [Online] Available at: https://www.iso.org/standard/9169.html
- European Medicines Agency, 2020. Quality of Water for Pharmaceutical Use. [Online] Available at: https://www.ema.europa.eu/en/quality-water-pharmaceutical-use
- The United States Pharmacopeia–National Formulary, 1985. 1231 Water for Pharmaceutical Purposes. The United States Pharmacopeia–National Formulary, 30(5)(USP29-NF24), p. 1744.
Related Content

pH in Medically Assisted Reproduction Procedures:Its Importance, Measurement and Control
Summary The intracellular pH (pHi) of gametes and embryos is modulated by a number of systems and, in human oocytes…

Cryoprotectants
Introduction Cryopreservation is integral to providing a safe, efficient, and comprehensive clinical service in assisted reproduction, encouraging elective single embryo…