Introduction
Some of us will still remember the ‘good old days’ when, every two weeks, we would neatly line up the containers of Analar grade salts and meticulously weight out NaCl, KCl, CaCL2, glucose, sodium lactate syrup, antibiotics, phenol red etc.
This would then be slowly dissolved (in a particular order) in ‘ultrapure’ water produced by a water system (which took many hours each week to sanitize and monitor), adjust its osmolality using a notoriously temperamental freezingpoint depression osmometer and finally filter it (using a peristaltic pump) into perhaps a hundred small flasks.
Alongside this, it was commonplace to test each media batch using a mouse QC model.
This involved the superovulation of mice using pregnant mare serum gonadotropin (PMSG) to obtain oocytes for IVF, or often 2-cell embryos which were then cultured in existing and new batches of culture media using a high blastocyst formation rate (>80%) as a marker of media ‘quality’ – the composition of which was optimized for the culture of mouse embryos, not human!
Fortunately, the world of IVF culture media has, of course, moved on hugely since the early days and virtually every IVF laboratory world-wide now source their culture media commercially from expert manufacturers.
Such complex culture media are now specifically formulated for human IVF and arrive in the lab with an assurance of quality, efficacy, and are ready to use.
However, it may be prudent to re-visit some of the basic ways in which commercially available culture media are used ‘at the bench’. With the increasing standardization of equipment and methodologies within IVF labs, the notion of ‘marginal gains’ is now often discussed within the embryology community and perhaps there are some marginal gains to be made in relation to culture media use?
On the assumption that the lab is using a reputable and process-appropriate suite of culture media, we can reasonably assume that the embryos’ nutritional needs are being met and that the embryos are living in an environment free from infection. But this environment needs also to be at the optimal pH and osmolality for the embryos to thrive – this article considers this in the context of the modern IVF lab.
Osmolality
Although the optimal osmolality for in vitro human embryo development is unknown, it makes sense that the osmolality of commercially available culture media is broadly in line with the osmolarity of the in vivo embryo environment (i.e. 280-290 mOsm/ kg). For example, the osmolality of SAGE 1-Step and global total LP is specified as 257-273 mOsm/ kg and 260-270 mOsm/kg, respectively.
The fact that culture media are often supplied with a slightly lower-than-physiological pH introduces the very important fact that evaporation of water from media will cause its osmolality to increase. It is this simple evaporation of water that needs careful consideration in the modern IVF lab.
It is commonplace nowadays to mitigate the effects of evaporation by using oil overlay (in addition, of course, to the advantages of temperature stability and protection from infection). However, it is a common misbelief that evaporation will not take place when oil overlay is used. The effect of evaporation through the oil overlay and in particular, the effect of oil volume can clearly be seen in Figure 1, where extremely high osmolalities are observed when a smaller volume of oil-overlay is used1. Recommended oil overlayer is minimum 2 mm.
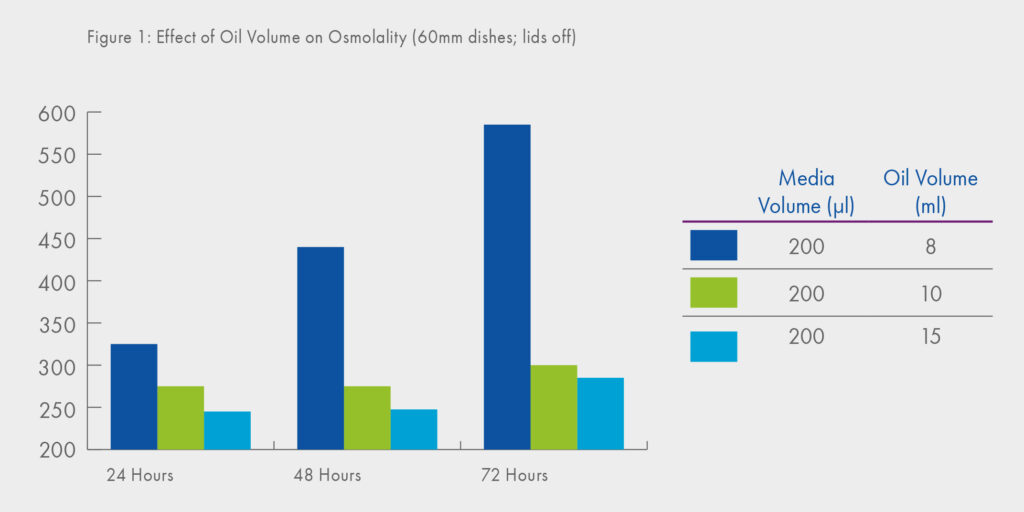
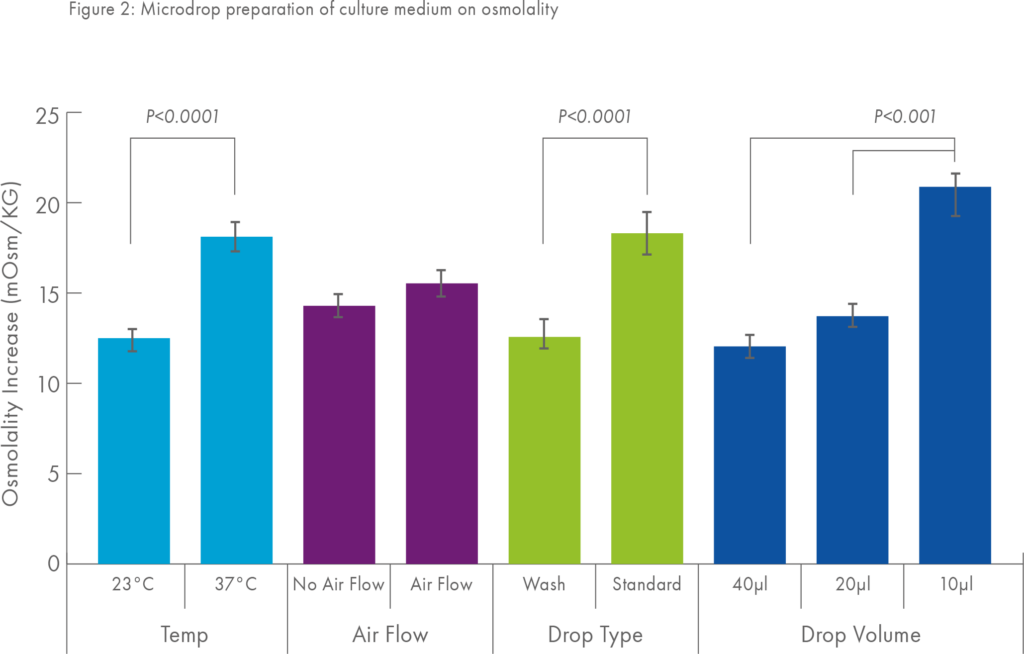
Furthermore, the data shown in Figure 1 were derived using a ‘large’ medium drop volume (200μl) leading to the second important consideration in terms of evaporation namely, that which might occur as small droplets of culture medium are being prepared.
This phenomenon has been elegantly demonstrated by Jason Swain2 and colleagues who investigated the effect of different ways of preparing microdrops (of different volumes) on their osmolality. Figure 2 shows, in particular, that preparing microdrops of small volume and preparing microdrops at 37°C rather than at room temperature can cause a significant increase in osmolality.
pH
Perhaps surprisingly, a robust RCT examining the effect of culture medium pH on human embryo development (and importantly live-birth outcome) remains absent from the literature, although it appears to be widely accepted that a pH of 7.2 – 7.4 is desirable. In the absence of a definitive human embryo culture pH it is worth examining some recent evidence which it seems difficult to ignore.
Swearman and colleagues3 have observed, using non-invasive polarized light microscopy that, in the mouse, the meiotic spindle is impacted by pH fluctuation. Although they found the effects reversible, should the same effects be present in the human, these data point strongly to the requirement for very close quality control of pH in the IVF lab.
As we are now all using commercially sourced culture media we are unable to adjust its composition to alter pH, having only the CO2 levels in our incubators as a variable factor at our fingertips.
Even so, culture media manufacturers will usually recommend a CO2 level at which they consider pH to be optimal and in these increasingly litigious days how many of us would dare to deviate from the information contained within a product data sheet?
Nevertheless, some basic physical chemistry continues to apply in relation to the pH/CO2 relationship and in terms of marginal gains this is worth re-visiting.
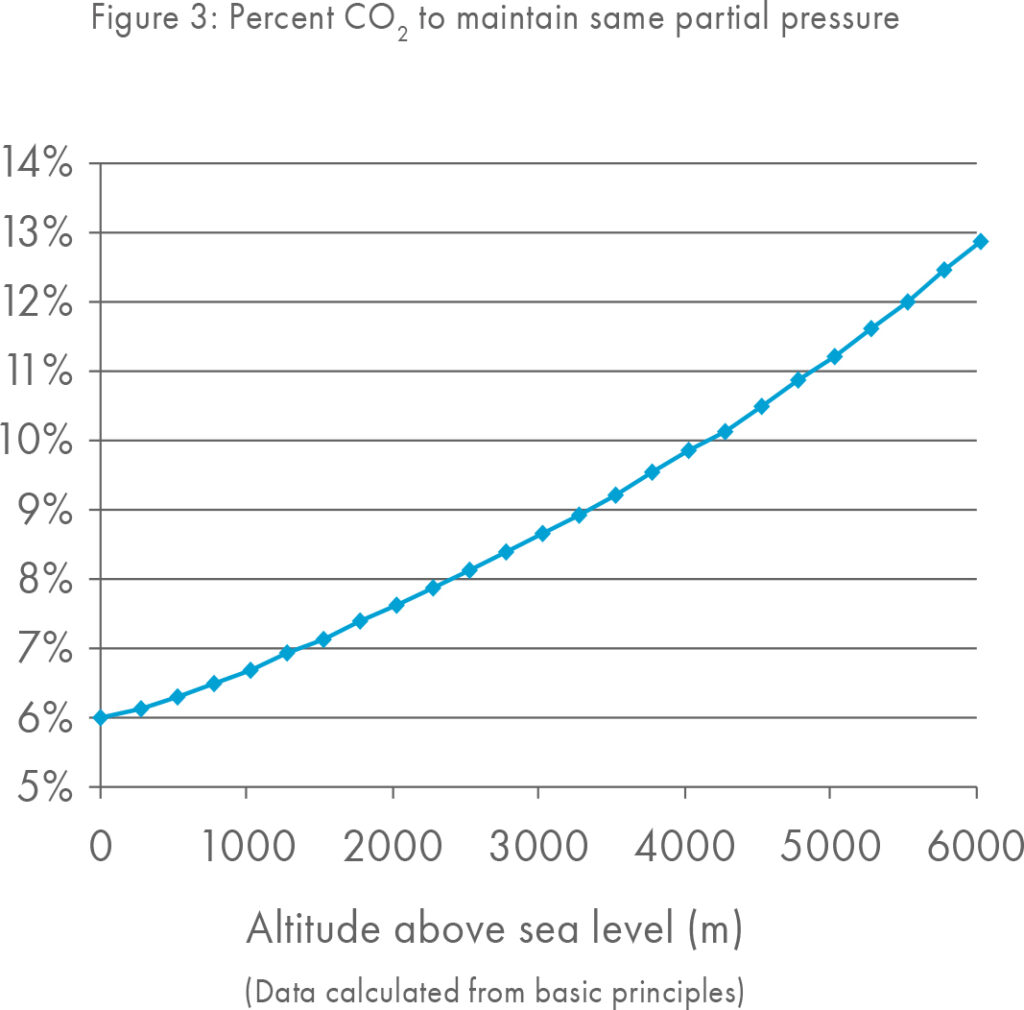
The CO2 recommendations given by manufacturers are generally only applicable for labs situated at sea level.
As the height above sea level increases the total atmospheric pressure, including the amount of CO2 present in the atmosphere decreases and as such labs at higher altitudes should increase their CO2 concentrations.
A lab at only 500 meters above sea level may wish to consider a small adjustment to increase the CO2 in their incubators. However, all labs are recommended to measure pH in order to culture with the optimal pH for a given media.
Equilibration time
It remains common place to set-up culture medium dishes allowing an overnight period for the oiloverlay and culture medium to equilibrate. Data has shown4 that overnight equilibration is effective in attaining optimal pH, but perhaps more importantly (in terms of marginal gains) demonstrate that equilibrating a dish for only a few hours will leave pH outside the accepted 7.2-7.4 range.
Summary
In conclusion, an IVF lab now has a choice of many commercially available culture media with subtle formulation differences, the quality of which in terms of manufacturing and QC process, far exceeds anything which could be produced ‘in-house’.
However, it is perhaps not quite as simple as using these products straight from the bottle and it remains essential to consider the basic physicochemical behavior of culture media and the way in which we use them in our own individual laboratory systems.
Finally, the data presented above suggest that we should not overlook the importance of routinely and accurately measuring pH and osmolality in our modern IVF labs and that by doing so marginal gains may be realized.
References
1. Barrie A, Kingsland C, Troup S. (2012) Hyperosmolality; a hidden threat? Hum Fertil. 15(S1): 1-26
2. Swain JE, Cabrera L, Xu X, Smith GD. Microdrop preparation factors influence culture-media osmolality, which can impair mouse embryo preimplantation development. Reprod Biomed Online. 2012 Feb;24(2):142-7. doi: 10.1016/j.rbmo.2011.10.008. Epub 2011 Oct 22
3. Swearman H, Koustas G, Knight E, Liperis G, Grupen C, Sjoblom C. pH: the silent variable significantly impacting meiotic spindle assembly in mouse oocytes. Reprod Biomed Online. 2018 Sep;37(3):279-290. doi:10.1016/j.rbmo.2018.06.022.
4. Steel T and Conaghan J. (2008) pH equilibration dynamics of culture medium under oil. Fertil.Steril. 89,4,S27