LABOR AND DELIVERY PORTFOLIO
Proven products designed with safety, confidence and preparedness in mind.
We are committed to providing proven and reliable devices that are simple and effective, empowering healthcare providers to feel prepared for any possibility. Our products are designed to reduce variability, so you and your team can act with confidence in every critical situation.
INDUCTION & LABOR
Help Ensure a Safe and Healthy Delivery from the Start
CooperSurgical’s induction and labor devices are proven and innovative solutions designed to reduce variability empowering you to act with confidence in critical situations.
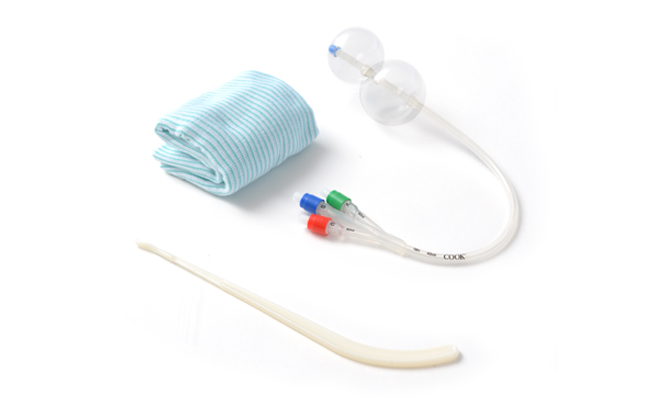

VAGINAL & C-SECTION DELIVERY
Be Ready for Complications During Delivery
CooperSurgical offers a variety of proven vaginal and c-section delivery products. These are reliable solutions designed to empower you to prepare for the unexpected during birth.
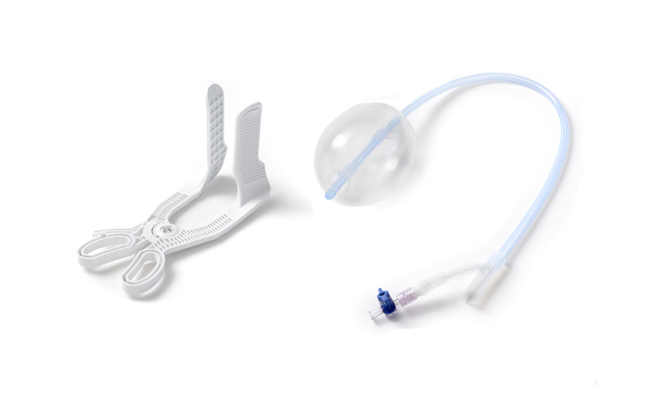
POSTPARTUM
Providing Devices for the Safe Care of New Mothers
CooperSurgical offers proven and innovative solutions designed to reduce variability empowering you to act with confidence in critical situations.

Contact your local representative for more information
IMPORTANT SAFETY INFORMATION
Fetal Pillow™is indicated to elevate the fetal head and facilitate delivery of the fetus in women >37 weeks gestation undergoing cesarean section at full dilation (with or without an instrumental delivery). The Fetal Pillow is contraindicated in the presence of an active genital infection. The safety and effectiveness of Fetal Pillow has not been established in the following: previous cesarean section, <37 weeks gestation, non-vertex presentation, intrauterine fetal death, pregnancy induced hypertension, intrauterine growth retardation, diabetes, major congenital abnormalities, chorioamnionitis, and multiple gestations. For detailed benefit and risk information, please consult Instructions for Use.
INSORB™ Subcuticular Skin Stapler is intended for use in the subcuticular closure of skin where an absorbable tissue fastener is desired for temporary tissue approximation. Contraindicated for: thin or thick tissue, and scar tissue where effective tissue capture cannot be achieved; if the needle path is obstructed, and when radiopacity or prolonged tissue approximation is necessary or desired. For detailed instructions and risk information, please refer to the Instructions for Use.
Read the Instructions for Use Prior to Use
The Single -Use Vacuum Devices and Cups (VAD) are indicated for use for non -reassuring fetal status, failure to delivery spontaneously following an appropriately managed second stage of labor, need to avoid voluntary expulsive efforts and/or inadequate expulsive efforts. No indication is absolute. CONTRAINDICATIONS: Do not initiate vacuum: non-vertex positions, suspected cephalopelvic disproportion, previous scalp sampling, suspected macrosomia, risk of shoulder dystocia, failed vacuum or forceps attempt, less than 34 weeks gestation, unengaged vertex, incompletely dilated cervix, need for active device rotation, suspected fetal bleeding abnormalities. WARNINGS: DO NOT exceed recommended vacuum levels. These instructions are intended as general guidelines. Practitioners should refer to current institutional and recognized guidelines that address VAD. Should only be performed or supervised by a trained and experienced operator.
For detailed instructions and risk information, please refer to the Instructions for Use.
MityOne™: MitySoft™ Bell Cup IFU, M-Style™ Mushroom™ Cup IFU
Mityvac™: Reusable Obstetrical Vacuum Pump IFU, Reusable Silicone Vacuum Extraction Cup IFU, MitySoft™ Bell Cup IFU, M-Style™ Mushroom™ Cup IFU
Mystic II™: Mystic™ II MitySoft™ Bell Cup IFU, Mystic™ II M-Style™ Mushroom™ Cup IFU
Footnotes:
* When compared to metal skin staples
References:
- Guardian Vaginal Retrator™ Directions for Use. Trumbull, CT. CooperSurgical, Inc.; 2002
- Cook™ Cervical Ripening Balloon with Stylet Instructions for Use. Bloomington, IN. Cook Medical; 03-2021
- MityHook™ Amniotome Directions for Use. Trumbull, CT. CooperSurgical, Inc.; 2018
- Mobius™ Elastic Retractor Systems Instructions For Use. Trumbull, CT. CooperSurgical, Inc.; 2018
- Schrufer-Poland, T. L., Ruiz, M. P., Kassar, S., Tomassian, C., Algren, S. & Yeast, J. D. (2016). Incidence of wound complications in cesarean deliveries following closure with absorbable subcuticular staples versus conventional skin closure techniques. European Journal of Obstetrics & Gynecology and Reproductive Biology, 206, 53-56
- Nitsche, J., Howell, C., & Howell, T. (2012). Skin closure with subcuticular absorbable staples after cesarean section is associated with decreased analgesic use. Archives of gynecology and obstetrics, 285(4), 979-983. https://doi.org/10.1007/s00404-011-2121-5
- Dresner, H. S., & Hilger, P. A. (2009). Comparison of incision closures with subcuticular and percutaneous staples. Archives of Facial Plastic Surgery, 11(5), 320-326
- Bron, T., & Zakine, G. (2016). Placement of absorbable dermal staples in mammaplasty and abdominoplasty: a 12-month prospective study of 60 patients. Aesthetic Surgery Journal, 36(4), 459-468
- Patel, V., Green, J. L., Christopher, A. N., Morris, M. P., Weiss, E. S., Broach, R. B., & Butler, P. D. (2021). Use of Absorbable Dermal Stapler in Reduction Mammoplasty: Assessing Technical, Quality-of-Life, and Aesthetics Outcomes. Plastic and Reconstructive Surgery. Global Open, 9(8), e3784–e3784
- Fetal Pillow™ Instructions For Use. Trumbull, CT. Inc.; 2023
- C SAFE™ Safety Scalpel Instructions for Use. Trumbull, CT. CooperSurgical, Inc.; 2015
- Bakri™ Postpartum Balloon Instructions for Use. Bloomington, IN. Cook Medical; 01/2020

