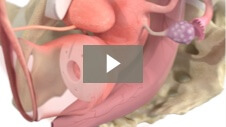
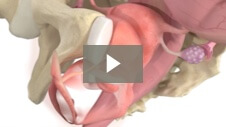
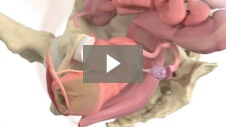
Endometrial ablation designed to significantly reduce heavy menstrual bleeding while preserving uterine cavity access1 Mara utilizes the expansive properties of water vapor to conform to each woman’s unique uterus, allowing you to treat more women1
- 93% of women report normal or no bleeding1
- 90% had an accessible cavity 4 years after treatment with minimal to no scarring1. This allows you to revisualize the endometrium and perform post-ablation diagnoses and uterine treatments as needed, even years later2
- 1 out of every 3 patients effectively treated in the Mara Pivotal Trial may not have been candidates with other ablation options1,*
- 99% of women experienced a significant improvement in quality of life3,†
* Patients with a broad range of anatomical conditions: Uterine cavity
lengths > 10 cm (up to 12 cm), cavities with certain types of myomas up to 4 cm, and in the presence of Essure®
† Based on patient reported Menorrhagia Impact Questionnaire (MIQ) scores
Please see Important Safety Information in the notes below
1. Mara Water Vapor Ablation System Instructions for Use
2. Johns D, et al. Post-Ablation Cavity Evaluation: A Prospective Multicenter
Observational Clinical Study to Evaluate Hysteroscopic Access to the Uterine
Cavity 4 Years after Water Vapor Endometrial Ablation for the Treatment of
Heavy Menstrual Bleeding. J Minim Invas Gynecol. Sept/Oct 2020; 27(6); 1273-1280.
3. Levie MD, Chudnoff SG. A Prospective, Multicenter, Pivotal Trial to Evaluate the Safety and Effectiveness of the AEGEA Vapor Endometrial Ablation System. J Minim Invas Gynecol. May/June 2019; 26(4); 679-87.
INDICATION FOR USE:
Mara Water Vapor Ablation System is indicated to ablate the endometrial lining of the uterus in premenopausal women with menorrhagia due to benign causes for
whom childbearing is complete.
IMPORTANT SAFETY INFORMATION
Pregnancy following the Mara procedure can be dangerous. Mara is not a sterilization procedure. Contraindications are: suspected uterine cancer, premalignant conditions of the endometrium, endometrial hyperplasia, prior classical cesarean section, transmural myomectomy or myomectomy performed immediately prior to Mara procedure, uterine length <6cm, prior endometrial ablation, active genital or urinary tract infection, PID, or systemic bacterial infection, an IUD in place, suspected hydrosalpinx, undiagnosed abnormal vaginal bleeding or are taking medications that thin the uterine muscle. Rare but serious risks may include perforation, infection, thermal injury, and serious complications in future pregnancies. Patients should contact their HCP if they experience any possible side effects, which may include cramping, nausea, abdominal pain, distension, vomiting, vaginal infection, and endometritis. Consult the IFU for detailed benefit and risk information.