RI Witness™ Laboratory Integration
Analyse your workflow
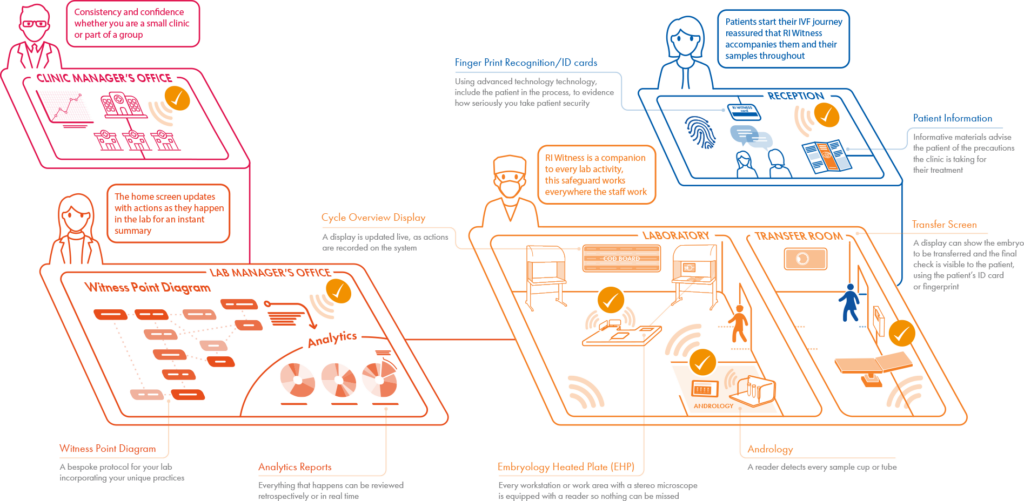
How RI Witness Integrates Into Your Clinic
Constantly communicating with your patient management system RI Witness, either visibly or invisibly, will have an impact at every level. Developed in response to industry voices, there are many features to choose from so you can select as many or as few as you require, leaving the option to expand at a later date. Each installation is tailored to your laboratory, and our installation team will manage your installation from start to finish.
RI Witness brings comfort to patients by constantly observing gametes/embryos at every process step of the IVF cycle, facilitating localization, and identification of all samples.
Availability
This product is available for sale in selected countries around the world.
“The integration of RI Witness into our daily routine was fast and simple and allowed for an improvement in system usage after a short period of time”
Dr Roberts Maggiulli, Biologist Laboratory Coordinator, Clinica Genera, Italy
Integration
We can fit embryology heated plates into all workstations from major suppliers; flush fitted integration is possible with K-Systems and ORIGIO cabinets.
Work areas can be connected through Wi-Fi or LAN cables to a central server which means everything works together and data updates in real time. Our software works with leading patient management databases. We also work closely with clinics and their database administrators on an individual basis to ensure smooth integration with RI Witness.
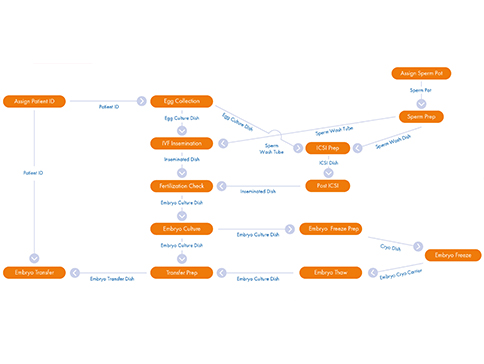
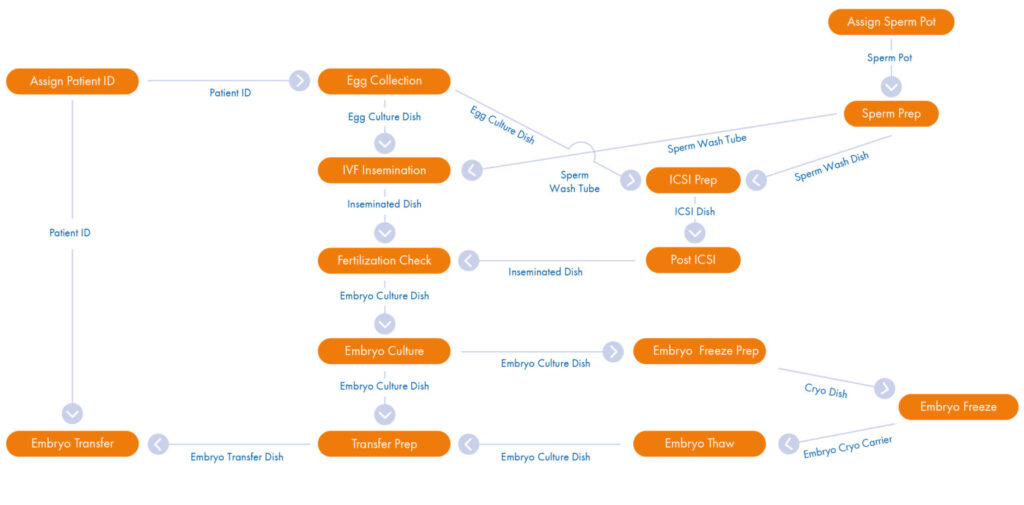
Your RI Witness Point Diagram
Mirroring your existing SOPs the Witness Point Diagram (WPD), created by your clinic’s laboratory management and our installation specialist, provides you with a comprehensive workflow overview. Each critical step carried out during every type of patient cycle is identified and captured in the WPD. Once tested and in place, the WPD allows the RI Witness system to record automatically real-time ‘who, what, where, when and how long’ data across all laboratory activity.
The WPD is flexible and future proof as you will be trained to be able to amend and evolve your protocols ongoing. Different types of cycles can be accommodated.

Implementation and Support
The logistics and practicalities of implementing a new system of any kind, can be extremely daunting for a busy IVF Clinic. However, our team of specialist installers will do everything possible to ensure a smooth transition.
They will work with you to communicate with clinic database providers to facilitate integration of data, if feasible.
Our installation team will support and guide the Senior Embryologists during the configuration of the workflow ensuring it is precisely how you want it. The nature of the RI Witness system is such that it forces very little change in daily tasks, which means that most normal users will see little difference in how they work. However, the team will ensure users understand the principles of the system and are trained to confidently use it.
Efficiency In The Lab
How can RI Witness make my lab more efficient?
Human double witnessing means a colleague must double check the identity of patient samples at critical points. This has been mandatory in the UK since 2004.
Since then, extensive comparisons have been undertaken to compare human, barcode and the RI Witness RFID systems. The evidence concluded that RI Witness was faster, more efficient, and ensured samples were out of the incubator for less time overall4 when compared to human double witnessing.
Product Specifications
| Work Areas | One work area required for each critical working location. Microsoft Windows based PC or Tablet needed for each work area. Readers available heated or unheated. RFID reader frequency: 13.56MHz |
| Barcode Compatibility (Traceability) | Compatible with GS1 barcodes (GS1-128) |
| Barcode Scanner (Traceability) | Compatible with USB (Keyboard wedge) fixed and hand held scanners |
| Camera Compatibility (Imaging) | Research Instruments’ DC1 & DC2, Analogue cameras |
| RI Witness™ Manager (Client Software) PC System Requirements | Operating Systems: Windows 11, Windows 10 |
| Server / Network Requirements | Microsoft SQL Server required (not supplied). Network Point required for each work area |
Product codes
The Order Codes for RI Witness™ will depend on your particular configuration.
Get In Touch With Us
We’d love to hear from you. How can we help?
Brochures, Catalogs & Flyers
RI Witness Brochure
RI Witness Brochure
RI Witness Product Guide
RI Witness Product Guide
RI Witness Comparison Booklet
RI Witness Comparison Booklet
RI Witness Patient Flyer
RI Witness Patient Flyer
RI Witness Patient Flyer – Same Sex Couple
RI Witness Patient Flyer – Same Sex Couple
Brady Printer Flyer
Brady Printer Flyer
1. Thornhill A, Orriols Brunetti X, Bird S, (2013). Measuring human error in the IVF laboratory using an electronic witnessing system. 17th World Congress on Controversies in Obstetrics, Gynaecology and Infertility, Lisbon. 1 Thornhill A, Orriols Brunetti X, Bird S, (2013). Measuring human error in the IVF laboratory using an electronic witnessing system. 17th World Congress on Controversies in Obstetrics, Gynaecology and Infertility, Lisbon.
2. Townsend N, Ah-Moye M, Bunyan K, Engley S, Evans D, Glover L, McClure A, Ogutu D, Richardson L, (2016). Can electronic witnessing with RFID tags safeguard patients and mitigate risk in an IVF laboratory?
3. Sanges F, Maggiulli R, Albricci L, Romano S, Scarica C, Schimberni M, Giallonardo A, Vattraino G, Ubaldi F, Rienzi L, (2013). Implementing an electronic witnessing system into a busy IVF clinic- one clinic’s experience.
4. Patel B, Schnauffer K, Gregoire, Kingsland CR, Troup S, (2013). An investigation into the efficiency of RFID electronic witnessing compared to manual witnessing.
5. Department of Health (2004). Independent review of the circumstances surrounding four adverse events that occurred in the Reproductive Medicine Units at The Leeds Teaching Hospitals NHS Trust. [online] London. Available at: http://www.who.int/patientsafety/information_centre/reports/Independent_review_Leeds.pdf [Accessed 12 May 2017].
6. Research Instruments Ltd. (2005). Test Report on Mouse testing of RFID Tagging System “IVF Witness”. Certificate of Analysis (Ectors, FJ, August 2005, GIGA Université de Liège,Belgium) Certificate of Calibration (Wragge-Morley, B. ETC, UK, May 2005) Certificate of Analysis (Pearce, J, May 2007, Embryotech, Wilmington USA)
7. Obradors, A. (2016). How can we mitigate the risk of error in the IVF Lab?
8. Forte, M., Faustini, F., Maggiulli, R., Scarica, C., Romano, S., Ottolini, C., Farcomeni, A., Palagiano, A., Capalbo, A., Ubaldi, F. and Rienzi, L. (2016). Electronic witness system in IVF – patients perspective. Journal of Assisted Reproduction and Genetics, 33(9), pp.1215-1222.
9. Universitair Ziekenhuis Brussels. (2020). IVF Witness in 2019, Internal Universitair Ziekenhuis Brussels presentation: Unpublished.
10. Mental health in the workplace. (2017). World Health Organization. [online] Available at: https://www.who.int/mental_health/in_the_workplace/en/.
11. Cooke, S. Low-risk laboratory management. In: Organization and Management of IVF Units: A Practical Guide for the Clinician. Eds: SD Fleming & AC Varghese. New York. Springer. 2016; Chapter 7: pp115-152.3. World Health Organization: https://www.who.int/mental_health/in_the_workplace/en/
RFID (Radio Frequency Identification) is a generic term for technologies that use radio waves to automatically identify objects. Labels with tiny microchips embedded in them are attached to all plasticware such culture dishes, test tubes and patient identity cards before being assigned to a patient. These microchips are read by the RI Witness readers. There is no need to directly scan the labels, the whole process is automatic and many tags can be read simultaneously.
RI Witness uses RFID tags and a reader. The reader sends out electromagnetic waves and the tag receives these waves. The RFID tag draws power from the field created by the reader and uses it to power the microchip’s circuits. The microchip then modulates the waves that the tag sends back to the reader and the reader converts the new waves into digital data.
RI Witness uses a radio frequency similar to the signals received by your car radio. We have organised exhaustive independent Mouse Embryo Assay (MEA) studies to demonstrate that RI Witness RFID does not have any detrimental effect on the development of embryos. These tests used radio waves which are 700 times stronger than those used by the actual product.
The most significant difference is that barcodes use line-of-sight technology. That is, a scanner has to “see” the barcode to read it, which means it is necessary to orientate the barcode towards a scanner for it to be read. Barcode systems therefore rely on the user remembering to confirm patients’ sperm, oocytes and embryos match. This can be prone to human error, and if not done correctly, can give a false record.
RFID, by contrast, doesn’t require line-of-sight. Tags can be read as long as they are within range of a reader. In addition, multiple RFID tags can be read at the same time, meaning that several items can be identified together. RI Witness uses this ability to automatically check everything that is brought into the working area.
RFID technology provides a level of security that can eliminate human identification errors. It monitors everything in every work area, every second of every day. It also offers an extremely large number of unique identities (UID). This means that each sample container can has its own UID and that procedures can be tracked in detail. The system knows exactly what has happened to the samples and which containers were used. The communication between tag and reader is very secure.
Procedures
Want unlimited First line support?
All our USA and Europe Customers get free unlimited first line support with a service contract.
Support & Compliance
Our global team is committed to providing the highest standards of service and support.

Batch Certificates
Use this tool to enter your batch number and download the corresponding certificate of analysis.

Service
We offer a range of contract options to suit your needs: preventative maintenance and service, reliable access to spare parts, product training, and online handling of service requests.
Get In Touch With Us
We’d love to hear from you. How can we help?